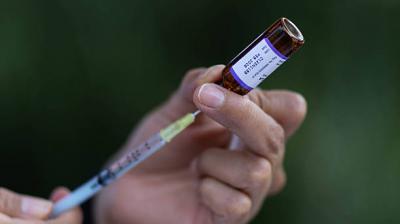
The Drug Controller General of India has approved the use of Itolizumab, a drug to cure psoriasis
New Delhi: The Drug Controller General of India has approved the use of Itolizumab, a drug to cure psoriasis, for “restricted emergency use” to treat Covid-19 patients with moderate to severe acute respiratory distress.
 CoronavirusDrugs Controller General of India Dr VG Somani approved an injection of the drug to treat cytokine release syndrome among coronavirus patients.
CoronavirusDrugs Controller General of India Dr VG Somani approved an injection of the drug to treat cytokine release syndrome among coronavirus patients.
“The approval was given after its clinical trials on Covid-19 patients in India was found satisfactory by the expert committee comprising pulmonologists, pharmacologists and medicine experts from All India Institute of Medical Sciences, among others, for treatment of cytokine release syndrome,” an official said.
There is no cure for the coronavirus, and treatment is symptomatic, though the recovery rate both in India and globally is high. On June 13, the Ministry of Health and Family Welfare approved the use of remdesivir for “restricted emergency use” in moderate cases.
 PhotoCountries across the world are also racing to develop a vaccine for the coronavirus. On July 2, the Indian Council of Medical Research directed clinical trial sites of Covaxin India’s first coronavirus vaccine candidate – to fast-track approvals and said that it planned to launch the vaccine for public use by August 15.
PhotoCountries across the world are also racing to develop a vaccine for the coronavirus. On July 2, the Indian Council of Medical Research directed clinical trial sites of Covaxin India’s first coronavirus vaccine candidate – to fast-track approvals and said that it planned to launch the vaccine for public use by August 15.